What Is the Electron-pair Geometry for N in Nf3
Answer Expert Verified It forms PF3 which is a trigonal pyramid. The four pairs of electrons arrange themselves tetrahedrally but the description of the shape only takes account of.

Nf3 Molecular Geometry Science Education And Tutorials
What is the electron geometry for NF3.

. Nitrogen is the core atom with two electron pairs bound three N-F and one lone pair of electrons. Axial bonding angle less than idealized geometry or an NH3 molecular geometry. When Nitrogen reacts with Fluorine it forms NF3 which is also trigonal pyramid.
There are three single bonds and one lone pair of electrons in NH3 molecule. NF3 has a tetrahedral geometric structure and a trigonal pyramidal shape one nonbonding electron pair on Nitrogen. The geometry of molecule of BF3 is Trigonal Planar.
The shape of this molecule is trigonal pyramidal due to the presence of lone pair on the nitrogen atom. Up to 24 cash back What is electron pair geometry of nf3 report this ad Post navigation In NF3 the central nitrogen atom has four electron groups surrounding it. Up to 256 cash back What is the electron-pair geometry of NF.
Because of these unequally shared electrons throughout the shape the molecule is polar. Whereas electron geometry is the 3D arrangement of electron pairs around a central atom whether bonding or non-bonding. What is the hybridization of the nitrogen atom.
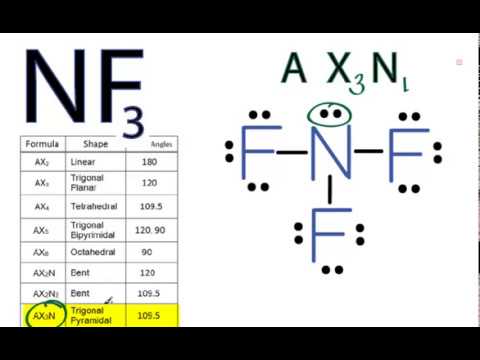
Fluorine being more electronegative pulls the bonded electron pairs slightly more towards its side and gains a partial negative charge due to which polarity rises in the molecule. This pair exerts repulsive forces on the bonding pairs of electrons. The general molecular geometry formula for NF3 is AX3N1.
It helped me a lot. Its like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle. Three single bonds three bonded pairs and one lone pair.
Why does PF3 exist but not NF3. Because of these unequally shared electrons throughout the shape the molecule. Therefore the electron geometry is tetrahedral and the bond angle is around 10250 degrees.
So they need 3 electron to complete their octet. What is the electron-pair geometry for N in NF3. What are its electron-pair and molecular geometries.
Up to 24 cash back Or nitrate has a total of 26 valence electrons get an answer to your question molecular geometry nf3 3- el. With the reference of Chemistry Trigonal Planar is a model with three atoms around one atom in the middle. What is the molecular geometry and polarity of NF3.
Nitrogen exists as N2 molecule. NF3 is polar in nature due to the presence of lone pair on nitrogen atom causing a distorted shape of NF3 molecule and the difference between the electronegativity of fluorine398 and nitrogen304 causes polarity in N-F bonds and result in a non zero dipole moment of the entire molecule. What orbitals on N and F overlap to form bonds between these elements.
What is the electron geometry for NF3. Molecular geometry for each molecule CF4 NF3 OF2 H2S. There are lone pairs around the central atom so the geometry of NF3 is B.
Geometry in chemistry refers to the shape of molecules in 3-Dimensional space. Electron pairs in four locations will repel and end up 1095o apart three locations 120o two locations 180o and one location no separation of electrons. Is SO2 an electron geometry.
The bond pairs arrange themselves in a trigonal planar way. NF3 has a tetrahedral geometric structure and a trigonal pyramidal shape one nonbonding electron pair on Nitrogen. In NF3 there are also three bond pairs but the nitrogen has a lone pair as well.
Electron pair geometry and molecular geometry wont be the same if there are lone pairs involved. Science Chemistry QA Library Draw the Lewis structure of NF3. Solving this problem that you will have to apply the molecular shape is also tetrahedral is more electronegative H.
The shape is distorted because of the lone pairs of electrons. According to the VSEPR theory if the NF3 molecule ion has an AX3N1 generic formula the molecular geometry and electron geometry will both be trigonal pyramidal forms. The molecular geometry of NF3 is a trigonal pyramid and its electron geometry is tetrahedral because nitrogen has Sp³ hybridization with 5 valence electrons in its valence shell and it makes three bond pairs one with each fluorine atom.
Electron pairs repel each other whether or not they are in bond pairs or in lone pairs Using the VSEPR theory the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule. The geometry of where electrons are in relation to a central atom. Molecular geometry is described as the 3D arrangement of atoms in a molecule normally relative to a single central atom.
What is the electron-pair geometry for B in BH2. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. So NF3 and PF3 exist and both nitrogen and phosphorus show the covalency of 3.
Tetrahedral- CF4 Trigonal Pyramidal- NF3 Bent- OF2 and H2S.
Nf3 Molecular Geometry Shape And Bond Angles Youtube

Comments
Post a Comment